So Simple,
It Works
Try IV Glove FREE Today
IVG Strapless

IV Glove 3.0
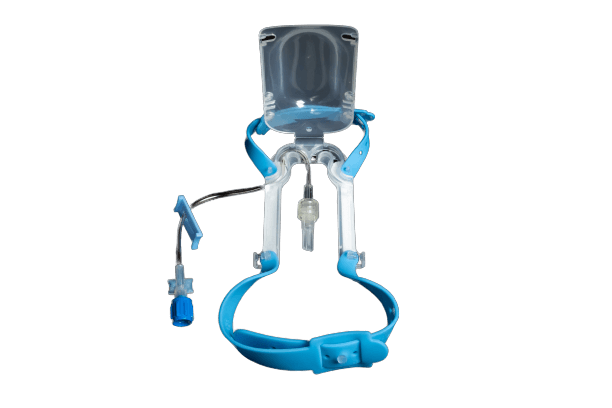
IV Glove

*The use of IV Glove is intended for improved intravenous care. The IV Glove 2.0 should only be used in accordance to hospital policies and procedures, as well as the healthcare providers completed e-learning module reflecting the understanding of the device purpose, and application technique*
Try the FDA Registered IV Glove Today
Frequently Asked Questions
The IVG looks like it only works for the top of the hand, but there are many other insertion sites. Do you have other IV securement devices?
The IV Glove is intended for insertion sites on the top of the hand. It is intended for PIVC’s on the hand for most young adults and adults of all ages. In addition to the IV Glove, we also have the IV Sleeve for the forearm, and IV Paediatrics for children.
Can peripherals with extended cannula be used with the IV Glove?
We are currently in the process of developing a model with a higher dome that can be easily used with an extended cannula/secondary port.
Can the IV Glove be used with central line catheter tubing, which are normally thicker than normal?
Yes, IVG is designed to work with all IV tubing including 3.0mm and 4.1mm.
Will the IVG work with all catheters?
Currently, our model works with most catheters. Our goal is to have models that work ALL types of catheters.
Is IV Glove reusable?
No, IV Glove is intended for single use only
Is IV Glove environmentally friendly?
The IV Glove is made with 100% recyclable materials.
Is IV Glove looking for partners/distributors?
Yes, please contact info@ivglove.com.

